Pharmaceutical manufacturers are major purchasers of processing systems and components. In general, valves, pumps, filters and other equipment components are higher priced per unit volume in the pharmaceutical industry compared to other industries. Stainless steel or other pristine materials of construction are needed to meet hygienic requirements. Performance requirements are high because the largest purchases are for components used to manufacture the product rather than in process cooling or water intake. A major new development is the discovery that cells from an individual patient can be extracted and then modified in a small processing system. The cells are then reinjected into the patient and are performing much better than drugs that are not individually tailored. This small, one-time system requirement is spurring the market for single-use pumps, valves and mixers.
Pharmaceuticals
The U.S. Trade Aministration’s 2016 Top Markets Report Pharmaceuticals defines pharmaceuticals (biopharmaceuticals, drugs, medicines) as “any substance intended for use in the diagnosis, cure, mitigation, treatment or prevention of disease or any substance (other than food) intended to affect the structure or function of the body. Drugs are produced in forms such as pills, tablets, capsules, vials, ointments, powders, solutions and suspensions.”
The report defines generic drugs as copies of innovative pharmaceuticals that contain the same active ingredients and are identical in strength, dosage form and route of administration. In the United States, upon either patent expiration or a successful challenge of relevant patents, a manufacturer can produce a generic drug as long as it meets Food and Drug Administration (FDA) approval and bioequivalence standards.
According to the U.S. Trade Admistration report, “generic drug companies typically focus on high volumes to earn profits, requiring efficient production methods and distribution chains.” Generics have seen significant growth. The large pharmaceutical companies have built plants in China, India and other developing markets to manufacture generics and compete in this segment of the market. This trend is significant for the future of the process equipment industry.
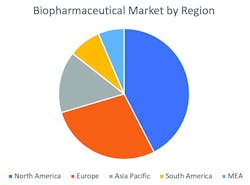
Figure 1. Biopharmaceuticals market revenue by region in 2017
Biologics
Accoding to the International Trade Administration, “biologics (biotech drugs, biological drugs, biopharmaceuticals) include a wide range of products such as vaccines, therapeutic proteins, blood and blood components and tissues. In contrast to chemically synthesized drugs, which have a well-defined structure and can be thoroughly verified, biologics are derived from living material (human, animal, microorganism or plant) and are vastly larger and more complex in structure. Biologic medicines are revolutionizing the treatment of cancer and autoimmune disorders and are critical to the future of the industry.”
Biosimilars
The International Trade Administration defines biosimilars (follow-on biologics) as versions of biologic products that reference the originator product in applications submitted for marketing approval to a regulatory body. Gaining regulatory approval in developed markets is far more complex for biosimilars than for chemical generics and may involve costly clinical trials. Those that succeed will also have to compete with the originator companies that are unlikely to exit the market. The biosimilars market is expected to increase significantly with an approval pathway now available in the United States. Prices of biosimilars may not be drastically cheaper than their patented counterparts.
Table 1. Oncology market segmented by end use
Market growth
Analysts are predicting a global growth rate for the pharma industry of 6.3 percent compound annual growth rate (CAGR) through 2022, up from 5 percent for the 2014-2020 period.
The overall $1.12 trillion market in 2022 in one estimate will rise at a faster clip during 2016-2020, then slow down a bit as major patent expirations take hold. Prescription sales will reach $1 trillion in 2022. Generics sales will increase to $115 billion in 2022 and constitute 10.2 percent of prescription sales at that point (only 0.3 percentage points more than it is now). The U.S. pharmaceutical market is projected to increase to $560 billion by 2022.
The largest growth market for processing components and systems is biopharmaceuticals. This industry generated revenue of more than $200 billion in 2017. Growth of more than 11 percent per year is anticipated for the next five years. The U.S. market share is now 40 percent of the total but will decrease slightly over the next five years. However, the growth in the U.S. is likely to be greater than 10 percent.
According to a Grand View Research report, the biopharmaceutical market can be segmented into four categories, namely biosimilar, enzyme, protein therapeutics and cancer monoclonal antibodies. The global biosimilar market has the highest market share and is expected to maintain its high growth rate for the estimated period. This market is driven by factors such as high pharmaceutical expenditure, and the presence of expensive drugs in this sector. Additionally, Grand View Research says the global protein therapeutics market is expected to register a high growth rate for the estimated period, owing to its efficiency in target therapy, which has little or no side effects. The global enzyme market and cancer monoclonal market are expected to grow at a steady pace for the forecasted period.
On the basis of geography, the market can be segmented in five key regions: North America, Europe, Asia-Pacific, South America and Middle East-Africa (MEA). North America has the highest market share for the estimated period owing to the presence of sophisticated health care infrastructure, growing geriatric population base and increased health care expenditure in the region. Asia-Pacific is expected to register the highest growth rate for the forecasted period mainly due to unmet market opportunities in the region.
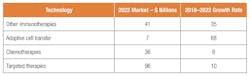
The market can also be segmented by end use. Oncology is a growing market. Cell therapy is only 1 percent of the $88 billion oncology market but by one estimate will grow from $1 billion to $7 billion by 2022. The total oncology market will grow to $180 billion in 2022.
The growth for adoptive cell transfer is likely to outstrip other segments.
A rapidly emerging immunotherapy approach is called adoptive cell transfer (ACT): collecting and using patients’ own immune cells to treat their cancer. There are several types of ACT but, thus far, the one that has advanced the furthest in clinical development is called CAR T-cell therapy.
CAR Ts are expected to account for 95 percent of the cell therapy market. According to a Research and Markets report, “Cell Therapy Manufacturing Market (2nd Edition), 2018-2030,” cell-based therapies have gained significant attention in the overall biopharmaceutical industry. The personalized nature of these treatment options render them highly specific and, according to a number of clinical studies, safe and efficacious. Such therapies are considered to have potential to address various unmet medical needs associated with the treatment of several types of physiological disorders and clinical conditions. Many pharmaceutical companies and investors have already invested significant capital toward the development and commercialization of such products. The Research and Markets report says sround 20 such therapies have been approved; recent approvals include Alofisel (2018), YESCARTA (2017) and KYMRIAH (2017). It is also worth highlighting that more than 500 cell-based therapy candidates are in different stages of clinical development and are being evaluated in more than 1,000 active clinical studies around the world.
From the perspective of process equipment design, the cell-based therapies that have been successful so far involve treatment of each patient with his or her own altered cells. Future research is focused on cell extraction and alteration from one donor for use by many.
Key players in the global biopharmaceutical market are Novartis, Pfizer, Merck & Co., GlaxoSmithKline, Abbott Laboratories, Amgen and Biogen.
The growth in sales of process equipment and components to this industry will continue at rates considerably above GDP.
Bob Mcilvaine is the president of the Mcilvaine Company in Northfield, Illinois. Mcilvaine provides market research and technical analyses on pharmaceutical process equipment and components. He may be reached at [email protected] or 847-784-0012, ext. 112.