Selecting the right materials to prevent exfoliation corrosion
Seasonal deicing salts are used worldwide when temperatures drop below freezing. While their primary use is on public roads and bridges, they are also sometimes used in large process plants, such as petrochemical complexes and tank farms. While the benefit is apparent, the consequences to equipment — including valves and accessories — are seldom accounted for and often go unrecognized.
As melted ice comes into contact with equipment, it carries with it aggressive elements, and the situation is aggravated when equipment is below road level, as with underground piping and sump pumps, for example. Accumulation of these water-concentrated salts leads to accelerated metallic corrosion, with one type being exfoliation corrosion.
Exfoliation corrosion can result from galvanic corrosion. While most metals are susceptible, it mainly affects aluminum alloys. For example, when brass and aluminum are brought into contact, the potential difference between the metals leads to accelerated aluminum corrosion. While the corrosion rate of aluminum is less than brass in salt-containing waters, the situation is aggravated in stagnant conditions as exposure time progresses due to galvanic coupling.
In some of these situations, equipment purchasers might focus mainly on initial cost, and may not be aware of the final application environment. Overlooking the potential for exfoliation corrosion in certain environments can lead to inappropriate materials of construction selection and improper coatings, resulting in premature equipment damage, with consequent downtime and substantial indirect added costs.
This article will present a use case where improper selection of materials of construction and coatings due to incomplete knowledge of the final application environment and metallurgy needs led to exfoliation corrosion, which could have been mitigated with proper selection.
Pressure regulator actuator casing corrosion
Two Fisher EZL Series regulators (Figure 1) by Emerson were installed in a North American natural gas distribution system on a 3-inch piping system.
These devices are pilot-operated, pressure-balanced, soft-seated regulators. They are designed for use in natural gas distribution applications, such as district regulating stations and commercial/industrial meter sets. They provide low differential, tight shutoff, long lifespans and smooth and reliable operation.
The regulator actuator casings were constructed of anodized aluminum alloy (type UNS A92014-T4) with brass bolts. The regulators were installed in encasings just below road level at various locations, but within the same geographical region in Canada. Seasonal deicing was frequently used during winter months, and both regulators had been in service for several years.
Localized corrosion was found during scheduled inspection and servicing by the municipality. Both regulator casings were taken out of service and submitted for evaluation. No record was kept indicating whether the brass bolts had been replaced while in service.
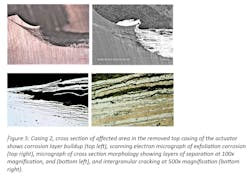
Visual observation revealed that both regulator casings experienced similar corrosion at the contact area between brass bolts and the aluminum casing. The remaining surface areas of the casings were free of corrosion (Figures 2 and 3).
The corrosion on both cases appeared to have emanated from the contact area of brass bolts with an aluminum casing, and it propagated on both sides. It is not clear if that particular bolt location had been replaced with the wrong material, or if so, when this occurred.
Evaluation
Samples from the casings were mechanically isolated, and then prepared for higher magnification and metallographic evaluation by applying Keller’s reagent, an acid base chemical used to reveal the microstructure of aluminum alloys. Both samples revealed identical corrosion morphology as shown in Figures 4 and 5. Corrosion was localized, initiating at the contact of the diaphragm level indicator connection and the casings body.
Reaction with the moistened environment, with its heavy concentration of aggressive anions, led to further accumulation of salt. This caused corrosion propagation along elongated grains, resulting in exfoliation corrosion of the casing. The casing microstructure is typical of aluminum alloy 2XXX series, which is composed of CuAl2 particles and particles of (FeMn)3 SiAl12 in an aluminum matrix. No microstructural anomalies were found that could have been attributed to corrosion initiation.
Rationalization
Corrosion initiated due to galvanic contact between the brass bolts and the anodized aluminum body of the pressure regulator. The contact area was subject to accumulation of environmental debris, which was agglomerated with moisture ladened with deicing slats. The resultant low pH surface moisture provided the necessary elements for galvanic corrosion to initiate after degrading of the anodized layer.
Once corrosion initiated, progression into the aluminum body was facilitated by its microstructural precipitates. Aluminum and aluminum intermetallic compounds (Al7Cu2Fe and Mg2Si) influence the material electrochemical behavior, but the extent of corrosion depends on precipitation location and concentration. At times, these compounds can become reactive, and aluminum can behave as an anode when coupled to copper and its alloys due to galvanic connections. This galvanic coupling leads grain boundaries to preferentially corrode via intergranular attack as copper intermetallic compounds are localized, making the area more reactive in relation to the microstructure matrix.
For example, it was found that an aluminum alloy containing at least 4% copper in solid solution exhibits an electromagnetic force (EMF) of −0.69 Volt when measured in a solution containing NaCl, but with copper present in the grain boundaries, the EMF was reduced to −0.84 V, where it becomes anodic.1 This behavior results in an increased corrosion rate, depending on the concentration of aggressive anions and oxygen availability. The maximum corrosion rate is expected when chloride concentration is at or near 2.3% weight.2
It was this galvanic effect that initiated corrosion and evolved into exfoliation. Galvanic corrosion does not need to have the typical two-metal contact with varying EMFs. Instead, a single metal can have microstructural elements with varying EMF, thereby fulfilling galvanic corrosion prerequisites.
Grain orientation and stacking faults
Exfoliation corrosion tends to progress more quickly through elongated grains than coarse grains. This is perhaps due to the greater energy of grain boundary density of elongated grains as compared to coarse grains, but research on this topic indicates there is no agreement as to why exfoliation corrosion occurs more aggressively in elongated grains. However, it is agreed that stacking faults affect surface properties and reactivity.
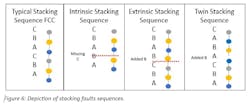
Deformed elongated grains result during some metal fabrication, specifically forging, rolling and drawing. As a result, recrystallization leads to stacking faults occurrence. This recrystallization occurs when deformed grains are replaced by a new set of grains that nucleate and grow until the original grains have been consumed and a new structure is created. There are three types of stacking faults that can develop during recrystallization: intrinsic, extrinsic and twin (Figure 6).
While a twinning stacking fault offers the advantage of increasing elongation during work hardening, thereby delaying plastic deformation, aluminum and its alloys tend to favor the intrinsic stacking fault due to vacancy clustering with a plane missing. Work hardening is the process by which material mechanical properties are changed, such as an increase in hardness.
Work hardening can result either deliberately (hammering, rolling, drawing, forging, etc.) or accidentally (unintended straining). Extrinsic stacking faults develop with an added plane. Plastic deformation is the irreversible change (yield) in shape resulting from applied loading. Prior to reaching the point of plastic deformation, the material is resilient enough to absorb applied loading, but without apparent change in dimensions.
A vacancy in a crystallographic lattice arrangement is an area with a missing atom or molecule, sometime referred to as a point defect or imperfection. Such a defect can alter material electrical and/or optical properties. During deformation, vacancies can populate in an area and become clustered.
The process of recrystallization causes deformed grains to be replaced by a new batch of flawless grains, which nucleate and increase in size until the original grains are replaced.
Aluminum alloy stacking faults can form during initial material foundry manufacturing stage, crystal growth, and plastic deformation. During plastic deformation, dislocations move because of crystal atom bonds breaking with applied load on a working plane. Clustering and accumulation of these dislocations resist applied loading, which contributes to increased rate of work hardening, and thereby increases in overall strength.
Aluminum and their alloys tend to have high stacking fault energies, which favors dissociation of dislocation into partial dislocations, with minimal separation. It is estimated (experimentally) that the stacking faults for aluminum of 137 millijoules per square meter3 is higher than nickel at 120–130 mJ/m2 4, copper at 40 mJ/m2 5 and stainless steel at 32.8 mJ/m2.6 For this reason, aged-hardened aluminum alloys tend to be prone to exfoliation corrosion. In other aluminum alloys, stacking faults become a precursor to hydrogen trapping, which leads to hydrogen embrittlement.7
While stacking fault effects are inevitable, proper manufacturing and postprocessing can alleviate undesirable mechanical and corrosion properties. One method is by induced twining.8 Another is by proper selection of alloying elements, which may contribute to reduction of stacking fault energies. While the first method is useful, it is usually not used due to its added cost and required processing time, with several iterations required to reach final desired properties. There are some claims of reversed uptake of the hydrogen effect (embrittlement) loss of ductility to induced ductility (ductilization) by means of staking fault energy reduction.9
Conclusion
A combination of factors contributed to the exfoliation corrosion discussed in this article, including improper selection of materials of construction and coatings, material intrinsic properties, application site geography, dissimilar metal contact, atmospheric/environment and secondary externally applied environment modifiers (deicing).
The most important method to prevent such corrosion is by selecting the right materials of construction and coating them properly. This helps isolate active metal surface from aggressive environments and limits immediate contact between dissimilar metals.
Proper materials of construction and coatings were used in the example given, but unexpected environmental changes degraded the protection level of the coatings. If the corrosion could have been caught early and an external coating covering the entire surface applied, propagation could have been prevented.
All these and other factors should be considered when purchasing pressure regulators and other valve accessories because proper material selection and preparation will increase service life, resulting in lower lifecycle costs, increased uptime and improved safety.
Ali Babakr, PhD, is a senior principal materials engineer for research and development at Emerson. Dr. Babakr previously held various positions with Emerson and other companies as a subject matter expert dealing with metallurgical and failure evaluation, material selection, and corrosion studies. He has field experience in the petroleum and petrochemical industries, and he participates in various industry subcommittees. Dr. Babakr holds Master of Science and Doctor of Philosophy degrees in metallurgy from the University of Idaho, and a Bachelor of Science degree in chemistry from Huston-Tillotson University in Austin, Texas.
References
1. Kavalco, Patricia Mariane; Canale, Lauralice, “Strength and Intergranular Corrosion of Aluminum Alloys: Effect of Cooling Rate.” Encyclopedia of Aluminum and Its Alloys, Totten, George E.; Tiryakiolu, Murat; and Kessler, Olaf eds., Sep. 2016, p. 2543
2. Ju, H.; Duan, J.; Yang, Y.; Cao, N.; Li, Y. “Mapping the Galvanic Corrosion of Three Coupled Metal Alloys Using Coupled Multielectrode Array: Influence of Chloride Ion Concentration.” Materials 2018, 11, p. 634.
3. Dodaran, Mohammad; Guo, S.M.; Khonsari, Michael; Shamsaei, Nima; Shao, Shuai, “A theoretical calculation of stacking fault energy of Ni alloys: The effects of temperature and composition.” Computational Materials Science, 2001.
4. C.B. Carter; S.M. Holmes, “The stacking-fault energy of nickel.” Philos. Mag. A J., Theor. Exp Appl. Phys. 35, 1977, p. 1161.
5. Janani, R Devi; Salman, Sameer Aman; Priyadharshini, K. Pavithra; Karthik, V.,” Effect of composition on the stacking fault energy of copper-nickel alloys using molecular dynamics simulations.” Materials Today: Proceedings, Volume 39, Part 4, 2021, p. 1796.
6. Woo, W.; Jeong, J.S.; Kim, D.K.; Dong-Kyu; Lee, Cheol Min; Choi, S.-H; Suh, J.-Y; Lee, S.; Harjo, Stefanus; Kawasaki, Takuro, “Stacking Fault Energy Analyses of Additively Manufactured Stainless Steel 316L and CrCoNi Medium Entropy Alloy Using in Situ Neutron Diffraction.” Sci Rep 10, 2020, p. 1350.
7. Zhao, H.; Chakraborty, P.; Ponge, D.; Hickel, Tilmann; Sun, Binhan; Wu, Chun-Hung; Gault, Baptiste; Raabe, Dierk, “Hydrogen trapping and embrittlement in high-strength Al alloys.” Nature 602, 2022, p. 437.
8. Liu, L.H.; Chen, J.H.; Fan, T.W.; Liu, Z.R.; Zhang, Y.; Yuan, D.W., “The possibilities to lower the stacking fault energies of aluminum materials investigated by first-principles energy calculations.” Computational Materials Science, Volume 108, Part A, 2015, p. 136.
9. Zen-Hao Lai; in, Yi-Ting; Sun, Yi-Hsuan; Tu, Jui-Fan; Yen, Hung-Wei, “Hydrogen-induced ductilization in a novel austenitic lightweight TWIP steel.” Scripta Materialia, 2002.