Best practice for evaporation processes
Evaporation is a process where a material moves from its liquid state to a vapor (or gas). The most common example of evaporation seen in everyday life is the transfer of water from the earth’s surface to the atmosphere as part of the water cycle. When liquids evaporate, and materials which they contain in solution or suspension are left behind, this becomes a useful process to concentrate solutions or to help separate materials.
Evaporation is different from dehydration or drying because the product is a concentrated liquid, rather than a dry solid. However, evaporation and drying processes can be combined: an evaporation process first removes the bulk of the water, and a final dryer eliminates the last bit, to obtain the final dried product. As evaporation plants are usually more energy efficient than dryers, it makes sense to combine the two technologies. Evaporation is different from distillation: a distillation process separates two or more liquids that have different boiling points (and which normally contain no solids). In contrast, evaporation eliminates water from a solution that contains dissolved and/or suspended solids, thus obtaining a more concentrated solution.
In general terms, the rate of evaporation depends on the temperature (the warmer it is, the faster the rate of evaporation), but the boiling point of a material varies with pressure (water boils at a lower temperature as pressure reduces), and so industrial processes often use a reduction in pressure to speed up the evaporation process.
Evaporation is used for different purposes in multiple industries and sectors. In the food industry, some products are concentrated to increase shelf life, reduce volume or weight, or reduce storage and transport costs. In contrast, in the pharmaceutical sector, evaporation is often used to create concentrated solutions that can then be dried to create powdered products. In each sector, the basic principles of evaporation remain the same — the removal of water (or another solvent) from a solution by converting that water or solvent into its vapor phase.
Different techniques, using different temperature and pressure conditions, can be applied in different situations. The type of evaporation that is most suitable for a particular purpose depends on many factors, including the nature of the solvent and the solution, the required end products, and the energy available for the process.
Types of evaporation equipment
Evaporation may be carried out in batches or as a continuous process. Evaporation consists of two elements: a heating phase (bringing the product to a boil) followed by an evaporation phase (where molecules leave the liquid phase and become gaseous). There are several types of equipment for performing these processes, including:
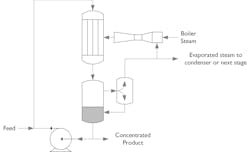
Jacketed tank evaporators (JTE)
These are among the simplest evaporators and are ideally suited to applications of small capacity and where capex is limited. The product is fed into a tank which has an external heating jacket in which the heating media flows. The product is raised to its boiling point and steam evaporates and exits from the top. For good heat transfer or if product fouling is likely, an agitator or scraper is installed to increase product turbulence and heat transfer rates. Jacketed tanks have less heat transfer area per unit of product volume compared to tubular or plate evaporators making them only suitable for relatively small evaporation tasks. However, there is no need for a recirculation pump, making them simple and easy to install.
Forced recirculation evaporators (FRE)
This type of evaporator was one of the first systems developed industrially and refined by HRS using our proven corrugated tube heat exchanger technology. In a FRE system the product is super-heated to a temperature above its boiling point. Upon exiting the evaporator, the product is introduced in a flash separation vessel where the pressure is lowered. Due to the reduction in pressure part of the product will flash off, and the concentration of the fluid in recirculation is increased. The flashed steam is then recovered by condensing it back to water in a condenser. Depending on the product, FRE can use plate, corrugated tubes or scraped surface heat exchangers.
The evaporated steam is condensed and collected in a condensate tank. A vacuum pump can be connected to the condensate tank to control the evaporation pressure. The evaporated-condensed steam can be used to preheat the incoming product, allowing for significantly increased levels of thermal efficiency.
Falling film evaporators (FFE)
In falling film evaporators, the product is introduced at the top of a vertical tube bundle, where it is evenly distributed and falls downwards as a thin film against the tube walls. On the outside of the tube a heating medium, normally steam, is applied to raise the temperature of the product and evaporation takes place at the liquid film surface. The vapor generated as the product is evaporated, travels down with the liquid film and the steam velocity helps to move the film along the surface of the tube wall.
This method offers several advantages: Falling film evaporation generally leads to very high levels of heat transfer, while the product recirculation flow rate required is far less than for FRE evaporators, resulting in lower power consumption for pumps. Finally, as evaporation takes place inside the evaporator tubes themselves, no temperature gradient is applied to the recirculating product.
The characteristics of falling film evaporators make them particularly suited for applications where the service fluid temperature is close to the evaporation temperature, such as with thermal vapor recompression (TVR) or mechanical vapor recompression (MVR) evaporators (see below for more details). Furthermore, residence times in FFEs are short, making them particularly suitable for heat-sensitive foods such as fruit juices and milk.
Improving evaporation efficiency
Evaporation processes can be optimized in terms of energy use, by reusing the energy (latent heat) contained in the water that is evaporated from the product, or the use of steam compression devices. We will explain three possible methods:
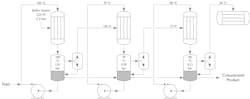
Multiple-effect evaporators
In a multi-effect evaporator, the evaporated steam generated in the first stage of evaporation is used as the thermal energy source for the next stage. This can be repeated several times over where the same quantity of steam is reused to evaporate multiple volumes of water. In such systems, the pressure of each consecutive stage is lower than the previous one, which also lowers the boiling point. For example, in a three-effect system, each evaporation stage takes care of one third of the total evaporation duty, and the three condensate streams (212°F, 167°F and 122°F) can be combined and used to preheat the raw incoming product prior to evaporation in the first effect.
A vacuum/pressure control system is required for precisely adjusting the evaporation pressure in each stage, making sure there is an optimal driving force between each effect. Multi effect evaporation is used to significantly increase the thermal efficiency of the process. For example, going from one stage to two stages reduce the thermal energy consumption by half. Adding a third stage reduces energy consumption by two third compared to a one stage process.
Thermal vapor recompression (TVR)
In a TVR compressor, part of the evaporated steam is mixed with ‘fresh’ steam from the boiler and this combined steam flow is then used as the thermal energy for that evaporation stage. The reuse of evaporated steam increases the energy efficiency of the plant. For example, if a TVR compressor is applied to a one stage evaporation plant, reusing 50 percent of the evaporated steam, this will then double the energy efficiency, compared to the system without the TVR compressor. TVR compressor can be combined within multi-effect evaporation plants further optimizing the energy consumption.
Mixing evaporated steam and boiler steam in a TVR device usually gives a net steam which is much closer the boiling point then pure boiler steam. This makes TVR processes difficult to apply for liquids with high boiling point elevation, and unsuitable for products with high viscosities and lower rates of heat transfer.
Mechanical vapor recompression (MVR)
Where thermal energy (steam or hot water) is not available but electrical power is, a lobe, or fan compressor can be used to recompress the steam which has been generated. By compressing the evaporated steam, the temperature and pressure is increased to a point where it can provide useful energy for evaporation. This way, the same kg of steam which is evaporated from the product is reused as the thermal energy source for the same evaporation stage.
The use of MVR compressors is one of the most economical evaporation techniques, with one of the lowest operational costs per ton of water evaporated. A final condenser is not required as the evaporated steam is condensed on the service side of the evaporator itself, also eliminating the cost of a cooling tower and corresponding cost of water for condensation. However, MVR systems often have higher capital costs due to the need for a larger evaporator due to the lower supply temperature. As with TVR systems, MVR compressor systems are less suitable for products with high boiling point elevations or products with increased viscosities
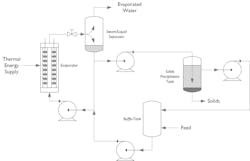
Zero liquid discharge (ZLD)
Perhaps the ultimate use of evaporation technology is for zero liquid discharge systems, which combine evaporation systems with solids precipitation or crystallization to achieve a net-zero liquid output from a process. The evaporator section concentrates the product as much as possible, typically to the point of saturation, before it is sent to the crystallization section where the solids are suspended and separated from the saturated solution. This saturated solution (supernatant) is recycled back into the evaporator and the process is repeated or continued.
For products with a steep solubility curve (high concentration at high temperature and low at low temperature) additional cooling to the solid’s precipitation tank will help the solids to precipitate quicker and settle out. ZLD systems are complex processes which are specifically designed for each application. In most cases product trials are required to establish the correct process parameters.
Arnold Kleijn is a Product Development Manager for HRS Heat Exchangers.