More certainty in corrosion prevention
Protective passive layers of stainless steel piping systems and tanks in chemical plants are at risk — and not only from the harsh conditions prevalent during production operations. Even in new systems, defects can be caused by grinding or by incomplete removal of temper colors after welding. TÜV SÜD Chemie Service participated in the assessment of a rapid test for corrosion developed by the German Institute for Materials Research and Testing (Bundesanstalt für Materialforschung und -prüfung, BAM). The tested method delivered valid information about the condition of passive layers on material surfaces.
Stainless austenitic chromium-nickel-molybdenum steel is the type of steel most frequently used in systems for the chemical industry and is composed of approximately 70 percent iron. Chromium is an alloying element with an essential role for corrosion resistance; at an alloy content of around 10.5 percent and higher, it forms a chromium oxide layer on the steel surface, protecting the underlying material from dissolution; however, this passive layer is only a few atoms thick. It is vulnerable and invisible. If the passive layer is not fully formed, there is a threat of corrosion. The same applies where imperfections are present in the material surface and prevent the passive layer from forming. The protective chromium oxide layer can reform in the presence of oxygen and moisture. However, permanent protection is only ensured if the conditions for repassivation are fulfilled. Important parameters in this context include adequate oxygen concentration, moisture and clean surfaces.
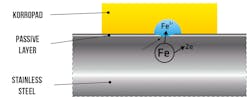
KorroPad rapid test: Indication in case of a defect in the passive layer. Graphic courtesy of BAM
Making imperfections visible
In the beginning, the material surface almost always looks perfectly clean and shining. Imperfections or defects in the passive layer are not visible to the naked eye; however, the level of corrosion protection achieved by passivation is critical in plant operation, ensuring the production facility can offer the required level of safety and cost effectiveness. After all, stainless steel surfaces are exposed to extreme conditions in their environment on a daily basis: Piping systems and tanks are in direct contact with acids, corrosive gases and other aggressive media, and imperfections in their passive layer can quickly result in damage events caused by dissolution of material, e.g., pitting corrosion. Given this, clarity with respect to corrosion resistance is paramount from the start; however, clarity is only possible if imperfections and defects in
passivation are visible.
Electrochemical measurement methods are routinely used in selecting safe and reliable materials. The possibility of pitting corrosion, for example, is identified using current-density potential curves. This is done in measurement cells in the laboratory, but also at localized points on the components themselves. Electrochemical measurements and salt-spray tests are reliable and proven methods for characterizing the corrosion resistance of the passive layer, but both methods have the disadvantage of being time-consuming and costly. Given this, experts were particularly interested in comparing KorroPad testing with conventional methods. The objective was to evaluate the new method and, if it proved suitable, to provide users with a simple on-site method.
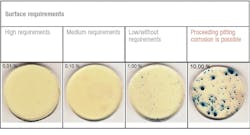
Indications of corrosion and requirements for surface corrosion resistance (in percent: areas of the tested surface showing a change in color). Graphic courtesy of BAM
Surface detectives show their true colors
If the passive layer is incomplete or damaged, ferrous ions are released at the defective points. This process forms the basis of this corrosion test method. The gel-like pads are filled with water and small amounts of sodium chloride and an indicator for ferrous ions. The indicator is potassium hexacyanoferrate (III), which is yellow to transparent in aqueous solutions and spontaneously changes to Prussian blue upon contact with ferrous ions. Clearly, visible blue spots appear in the light-yellow pads, indicating the points on the surface of the steel where the protective passive layer is not present or was unable to form properly.
Clear test results in 15 minutes
This corrosion test method is a non-destructive and, above all, simple and rapid test method. It enables the surfaces of pipe systems and tanks to be tested for corrosion resistance before they are installed in a chemical plant. As a further advantage, the method does not require any previous knowledge in the field of corrosion or electrochemistry. Three pads are used per test. The pads are placed on the steel surface and pressed down. Before the test, the surface to be checked must be cleaned with acetone or alcohol. The pads can be removed after only 15 minutes using a plastic spatula. They are then placed on a plastic carrier film. Scanning or photographing the test result is recommended for the purposes of evaluation and documentation. If the test identifies a corrosion risk, the experts will consult with the plant managers and agree on the necessary steps. The safety of the plant and the safety and health of production staff are always top priorities in this context.
Indication of corrosion in a pipe with longitudinal weld as delivered. Graphic courtesy of TÜV SÜD
Rapid test in field comparison
This rapid test is primarily a surface-specific test method. It can be used on all relevant stainless-steel types as demonstrated by TÜV SÜD Chemie Service in comprehensive field tests. Tests were carried out on austenitic chromium-nickel-molybdenum steels. The pads delivered reliable indications in all tests involving temper colors after welding. Even insufficient electrochemical cleaning or polishing using devices designed for this purpose or mechanical treatments (such as brushing the weld seams) resulted in indications. In these cases, temper colors had evidently not been adequately removed, preventing the passive layer from reforming completely. Parallel to testing, TÜV SÜD Chemie Service carried out local electrochemical measurements for comparison. The results showed the pitting corrosion potential was lower at the points where pad testing had revealed indications so that increased corrosion probability must be assumed.
As a further advantage of this method, the rapid test showed whether passivation had fully developed after grinding, etching and other cleaning steps, confirming that no further problems should be expected during operation. The experts were also able to prove that the method is suitable for quality assurance, because defects and imperfections on the exterior of pipes with longitudinal welds could be demonstrated clearly.
On the safe side
By performing numerous tests and applications in the field, TÜV SÜD Chemie Service was able to prove that the method is suited for characterizing the corrosion resistance of the passive layer. The rapid test can also be used to test the surface of steels, both in as-delivered condition and after processing, to investigate the risk of corrosion. This enables industrial trade operations to defend themselves against costly warranty claims. Last but not least, corrosion is more than merely a visual issue: Stainless steels are frequently used in manufacturing tanks to hold hazardous materials or for complex production systems. Given this, first and foremost the rapid test also supports plant safety.
Dr. Helga Leonhard is test engineer, Materials Engineering and Testing, at TÜV SÜD Chemie Service GmbH. She may be reached at [email protected].
Dipl.-Ing. (FH) Jens Lehmann is research associate at BAM – Bundesanstalt für Materialforschung und -prüfung. He may be reached at [email protected].