Controlling chemical vacuum processes with direct sealing diaphragm valves
The use of vacuum technology to create Edison’s electric light bulb in 1879 is widely known. Since then, researchers have discovered thousands of other ways to take advantage of running a process in vacuum. While these may not be as famous as Edison’s invention, modern vacuum processes have had an impact on the world that is difficult to overstate. The manufacturing of smartphones, solar panels and silicon chips, for example, would not be possible without vacuum technology.
Over the past decade, the use of vacuum technology for chemical processes has also increased dramatically. Vacuum plays an integral role in life-saving pharmaceutical developments, in laboratory research, in food and beverage processes, and in environmental efforts such as polymer devolatilization.
Some of these new chemical vacuum processes involve harsh chemistries, which present unique and sometimes quite difficult challenges. Due to the hazards involved with corrosive chemicals, process components must be chemically compatible, as well as extraordinarily accurate and precise. For any given vacuum application, additional process conditions such as high temperatures or automation requirements can add further complications.
As a result, traditional methods of controlling vacuum are not always suitable, and alternatives must be considered.
One relatively new approach for providing vacuum control of chemical processes is the use of direct sealing diaphragm valves.
A classic example: vacuum distillation
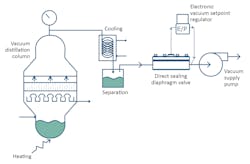
To illustrate possible reasons for using a direct sealing diaphragm valve to control vacuum in chemical processes, consider the relatively familiar process of vacuum distillation.
Almost everyone understands the method used to make whiskey and other distilled beverages: because water and alcohol have different boiling temperatures, alcohol boils off earlier than water, allowing the alcohol to be collected.
But what happens if a chemist or engineer needs to separate two delicate compounds that are heat sensitive? This situation happens frequently in the manufacturing of chemicals or pharmaceuticals with long chain molecules or polymers. Often, the best solution is to lower the boiling temperature by reducing the pressure over the liquids.
In this situation, vacuum distillation is used to lower the pressure above a liquid to less than its vapor pressure, allowing the most volatile liquids to be selectively boiled off and distilled. This is particularly useful if the temperatures required for a fluid to boil at atmospheric pressures would be hot enough to damage sensitive molecules through polymerization or by thermal cracking. Precisely controlling the vacuum pressure is critical because the mixture being distilled may contain several liquids with close boiling points. Accuracy in controlling the vacuum allows much higher selectivity in the distillation.
Real-world vacuum applications and challenges
Matthias Bogar manages the German division of Pressure Control Solutions, a fluid control specialty company that works extensively with research scientists and industries throughout Northern and Western Europe. He has years of engineering experience designing vacuum distillation systems.
According to Bogar, one of the most common applications for vacuum distillation is recycling or refining solvents. Vacuum distillation units are also used for quality control of crude oil and crude oil fractions as well as extraction of essential oils.
“In addition, for pharmaceutical manufacturing, a special thin film and short-path evaporators are used for vacuum distillation under very low vacuum,” Bogar said. “These are often used for the production of hormones, vitamins or other proteins that are easily destroyed by high temperatures.”
Bogar says that three challenges to consider when designing vacuum distillation systems are Flow Coefficient (Cv) range, precision and chemical resistance.
Cv range: The flow restriction required by a process can be described by its Cv, which is a function of flow rate, differential pressure and gas specific gravity. During the start-up of a vacuum distillation system, a large Cv is required to remove the atmosphere from the system. When the system is at its target vacuum, on the other hand, only minimal gas passes through the vacuum regulator — most of the product stays in the system either being condensed at the column head (distillate) or in the evaporator as residue. Only non-condensable gases are being pulled through the regulator and into the pump. At that point, the low Cv capability of the regulator is important.
In addition, batch distillation systems can involve multiple steps; for example, in some systems, distillate needs to be discharged, usually by separating a distillate receiver that can be ventilated to discharge product.
“All in all, there are many scenarios during a distillation where the required Cv of a vacuum control valve can change rapidly,” Bogar said.
Precision: When separating two products with similar boiling points, even a couple mbar of fluctuation can easily contaminate the distillate. “An excellent example is the refining of rose oil for cosmetics,” Bogar said. “In one instance, one small fraction in the oil was suspected to be carcinogenic. To be able to distill this out of the product required high precision because you needed to be sure none of the potentially carcinogenic fraction was left in the product, but at the same time you didn’t want to waste product in that fraction.”
Chemical resistance: Working with harsh chemistries always involves risk that must be managed as safely as possible. “Even though almost every distillation system that I built was equipped with a cold trap placed in front of the vacuum control valve and the pump to protect them from acid vapors, there is always a chance that those vapors will pass through the cold trap. Especially, that can happen with non-condensable gases,” Bogar said. “It is basically a question of the capacity of the cold trap, the amount of vapor and velocity. I have seen high-grade stainless steel control valves that were severely corroded.”
Traditional approaches
According to Bogar, several traditional approaches are possible when designing a chemical vacuum process. A combination of two solenoid valves — one to serve as a breaker by bleeding in air/nitrogen and one to throttle flow to the vacuum pump — can work for vacuum down to 0.1 mbar. For deeper vacuum, it can be easier to work with a vacuum breaker such as a small bleed of nitrogen. In certain low-cost systems, it can be possible to use one solenoid valve in combination with a manual throttle valve, which must be adjusted depending on the vacuum pressure. In this case, the actual control is provided by the solenoid valve.
Complex challenges
Complex chemical vacuum systems often present combinations of challenges that cannot be addressed with traditional methods. For example, most traditional vacuum regulators do not offer the accuracy required to selectively distill fluids with close boiling points. Moreover, many of the vacuum regulators that are sensitive enough to do so cannot withstand the corrosive chemistries or elevated temperatures often encountered in vacuum distilleries.
A direct sealing diaphragm valve, on the other hand, can provide both accuracy and durability. In addition, it provides superior precision, offers an extremely wide Cv range and works well with automation, which is increasingly common in modern processes.
How a direct sealing diaphragm valve provides more versatile vacuum control
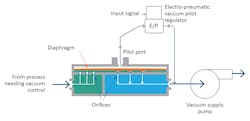
A direct sealing diaphragm valve controls vacuum pressure accurately and can hold the vacuum precisely at the required set point regardless of fluctuations in the system flow rate or variations in the supply vacuum pressure. It can do this because a responsive flexible diaphragm is the only internal moving part.
The diaphragm is nearly frictionless in its movement and does not have the accuracy-robbing hysteresis and static friction that traditional ball, gate or butterfly vacuum valves often have. The diaphragm has extremely low mass and will respond to changes in system flow rate within milliseconds.
This type of valve uses multiple orifices sealed by a flexible diaphragm rather than a single large valve seat. It is dome-loaded by a pilot regulator setting the vacuum setpoint on top of the valve. As flow requirements change, the diaphragm moves a few millimeters to open and close over none, some or all of the orifices, providing instantaneous and frictionless control. The pilot setpoint pressure may be set with a manual or electronic pilot regulator, which makes the valve well-suited for automation.
“The rangeability, sensitivity and compatibility with highly aggressive media make the direct sealing diaphragm valve an interesting option for many vacuum distillation setups,” Bogar said. “Sensitivity is important when you need precise control to maintain purity of the product with no contamination from other fractions. And the simple design definitely helps with cleaning and maintaining the valves. Many systems become contaminated quickly. It’s ideal to distill with solvent vapors directly through the valve to do a clean-in-place type of procedure, but the simple design of the direct sealing diaphragm valve allows you to quickly open the valve to clean or change parts.”
Beyond distillation
The same advantages that direct sealing diaphragm valves offer for vacuum distillation also apply to other chemical vacuum processes such as evacuating gases in process vessels, processing high temperature fluids and accommodating complex flow systems. The following examples show how this technology has been successfully used in specific situations:
A candle manufacturer uses a direct sealing diaphragm valve to control vacuum on a wax processing vessel. Vacuum setpoint control in the vessel is important for candle quality. If the vacuum increases above setpoint, the aromatics begin to boil off.
A research lab at a large technology company uses a direct sealing vacuum valve to evacuate gases in a controlled manner during solvent mixing. Application conditions include high temperatures and solvent handling, which are typical reasons for choosing direct sealing diaphragm valves.
A semiconductor material supplier installed a direct sealing diaphragm valve at the outlet of a crystal growth chamber to maintain a proper growth environment in the range below 5 torr. The flow regime is highly sensitive and earlier methods of controlling vacuum were inadequate. The vacuum valve was sized to account for this difficult application. In addition to the sensitivity required for this process, the vacuum equipment had to be compatible with the process gas.
Jeff Jennings, PE, is founder and president of Equilibar, which designs and manufactures direct sealing diaphragm valves for research and industrial applications. Jennings has more than 30 years of engineering experience in a variety of industries, including 16 years with The DuPont Company. He holds several international patents and continues to do active research in the field of fluid controls. He may be contacted at [email protected].