Enhance clean-in-place operations through optimized conductivity measurement
Professionals in the food and beverage, pharmaceutical and life sciences manufacturing industries know the critical role of clean-in-place (CIP) operations. The ability to clean a processing system including tanks, pumps, valves, filters, heat exchangers and process piping without having to disassemble any part of the system or shut down operations is essential to product safety, quality and elimination of cross-contamination. CIP also assures efficiency, shorter time to market and cost-effectiveness. Achieving these benefits, however, requires tight control of the CIP process. The right amount of cleaning solution must be used to be effective but removing that cleaning solution thoroughly and keeping it out of contact with the product is equally as important.
It is interesting that the measurement technology used to assure efficient and effective CIP is one of the most straightforward and time-honored — conductivity analysis. Using conductivity measurement appropriately and effectively in CIP, however, isn’t as straightforward. Plant managers and personnel need tips, techniques and best practices for the selection and application of conductivity technology.
Basics of CIP
The primary goals of a CIP system, regardless of industry, are to maximize safety, avoid cross-contamination and increase processing speed. Most CIP systems are multi-tank configurations used to allow for the recycling of water and possible regeneration of cleaning chemicals. Tanks in the system hold different quality grades of water, such as deionized water, water for injection and reverse osmosis (RO) water.
A typical CIP process contains:
- A pre-rinse with RO-treated water, consisting of three bursts— each of a one-minute duration,— to remove the bulk of the soil load
- A continuous 30-minute wash of alkaline detergent at 180°F (82°C)
- A one-minute rinse with RO-treated water
- A 30-second rinse (sometimes 10 minutes or more is requisite) of nitric or phosphoric acid solution at 150-180°F (65-82°C)
- A two-minute rinse with RO-treated water to remove the phosphoric acid residues
- A final one-minute rinse with deionized water
Additionally, in pharmaceutical and life sciences, the CIP process is harsher and involves multiple cycles, including an initial and final drain step, a pre-rinse, a sodium hydroxide wash and a post-rinse. Rinse and wash cycles vary from five minutes to one hour. The process may include a "sanitize" cycle to reduce the levels of bacterial contamination using strong oxidants such as hydrogen peroxide, ozone, chlorine dioxide or other chlorine-containing compounds.
When the CIP process is initiated, pre-rinse water is sent through the circuit and "chases" the product. A timing sequence based on distances and flow rates will switch the valves at the proper time to minimize the interface between product and rinse water. Over 90% of the product residue is removed during pre-rinse to minimize the use of washing chemicals. Proper cleaning (as determined by the regulatory requirements) is a function of detergent strength, cleaning time and temperature.
Figure 2. Accurate analysis across a wide conductivity range is vital.
Why conductivity analysis?
Conductivity is a measure of how well a solution conducts electricity. To carry a current, a solution must contain charged particles, or ions. Most conductivity measurements are made in aqueous solutions, and the ions responsible for the conductivity come from electrolytes dissolved in the water. Salts like sodium chloride and magnesium sulfate, acids like hydrochloric acid and acetic acid and bases like sodium hydroxide and ammonia are all electrolytes. Even though different electrolytes have different conductivities, the conductivity is not species-specific. It measures the total concentration of ions in solution and cannot distinguish one electrolyte or ion from another.
Beause the various cleaning solutions used in CIP are more conductive than the water used for flushing and final rinsing, conductivity can be used to monitor the various cleaning steps and the final rinse. Each cleaning solution’s flush is typically followed by a water flush, so each step of the cleaning process will appear on a strip chart as a series of conductivity increases. The progress of the final rinse can be followed as a decrease in conductivity until it drops to the conductivity of the rinse water, which indicates that all the cleaning solutions have been washed away.
To provide detergent of the proper concentration for each CIP circuit and to validate that cleaning was done at the proper detergent strength, conductivity measurement is applied to the returning acid and caustic. These conductivity measurements are proportional to the concentration or solution strength and are recorded by control systems for validation. The fluids are often partially neutralized during the CIP process, and additional concentrate dosing is required. Conductivity measurements indicate when sufficient concentrate has been added to the appropriate tanks.
Conductivity measurement is a cost-effective way to monitor the CIP steps. It is highly effective at detecting the interface between cleaning solutions and the product so that the valves can be switched at the appropriate time to minimize the interface between the two and any resulting product loss. Conductivity measurement can also determine the interface between cleaning fluids and rinse water to minimize CIP time while meeting compliance requirements.
Figure 3. Toroidal conductivity sensors are effective in solutions with high conductivity, are corrosive, or have suspended solids.
Challenges to conductivity
Conductivity measurement provides a cost-effective way to monitor and validate CIP, but it’s not without challenges. The stringent regulatory requirements and assurance of public safety mean that measurement of CIP processes must be very accurate and precise. There are a number of factors that line up against that assurance. The high cost of the caustics also calls for precision measurement so that none are wasted.
The caustic and challenging nature of the environment in which the conductivity sensor must work means the sensor used has to be highly robust to withstand the heat and chemicals of the process. At the same time, the sensor must be approved for use in food or pharmaceutical products. One of the major requirements for equipment and sensors that come in contact with the process is that they be sanitary in design, with USP class VI and FDA 21 CFR-compliant material and have 3A or other hygienic approvals where applicable.
An even greater issue is rangeability. A significant challenge in CIP conductivity analysis is that the process involves two distinctly different conductivity measurement ranges — one for moderate-to-high concentrations of acid and caustic, and the other for the purified rinse water. Therefore, any conductivity sensor used must be highly sensitive and accurate over the high conductivity range of CIP.
The final and often most significant problem in implementing conductivity measurement in CIP is one that confronts all plant managers regardless of industry — the rapidly changing workforce. Newer personnel often don’t have the level of experience or expertise with measurement technology that their predecessors did. Problems like difficult installation of the analysis system and unintuitive sensor setup and operation can lead to inaccuracy in measurement.
Optimizing conductivity in CIP
Choose inductive
There are two types of conductivity measurement: contacting and inductive. The choice of which type to use depends on the amount of conductivity, the corrosiveness of the liquid and the quantity of suspended solids. Contacting sensors bring the measurement electrodes into direct contact with the solution to be measured, which can foul or corrode the electrodes if exposed for a long period of time.
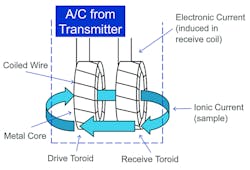
Inductive sensors have non-contacting electrodes and are often called toroidal, because they use toroidal transformers isolated from the process. One toroid acts as a transmitter and the other as a receiver. The transmitter toroid produces an electric current in the process solution that induces a voltage in the receiver toroid. The strength of that induced voltage is directly proportional to the conductivity of the solution. The toroids of inductive sensors do not need to touch the sample. Thus, they can be made of robust materials, allowing the sensor to be used in solutions that would corrode metal electrode sensors. Also, because inductive sensors tolerate fouling, they can be used in solutions containing high levels of suspended solids. As long as the fouling does not appreciably change the area of the toroid opening, readings will be accurate. By contrast, even a light coating of deposit on a contacting sensor will cause an error. Generally, the inductive method is better when the conductivity is high, the liquid is corrosive, or suspended solids are present as is the case in CIP applications.
Toroidal sensor technology seldom requires cleaning and features smooth surfaces with no crevices, unlike contacting conductivity sensors. Therefore, the toroidal sensors don’t collect or harbor residue or trap microorganisms. Coating and plugging are virtually eliminated. This makes toroidal sensors the ideal choice for CIP installations because they can be made sanitary by design. They can be manufactured of USP Class VI-compliant material, minimizing product contamination. Plus, these materials also resist corrosion in the caustic and high temperature environment of the CIP process, withstanding temperatures up to 266°F (130oC).
Choosing conductivity technology
When choosing an inductive conductivity sensor, choose for rangeability. Not all sensors are good choices for CIP. Wide rangeability means the sensor is accurately measuring from clean water up to the highest conductivity fluids. CIP sensors must allow for accurate measurement in the very high conductivity ranges of the application. Inductive sensors are ideal for measuring solutions having high conductivity, since these solutions produce a large, easily measured induced current in the receiver coil. At the same time, not all sensors offer accuracy in the lower ranges, so careful selection is essential.
Figure 4. Rosemount 225 PUR-Sense toroidal conductivity sensor from Emerson
The addition of contacting conductivity
While contacting conductivity sensors aren’t appropriate for measuring the high conductivity caustics of CIP, they do provide extremely rapid response to the rinse water. The response time of these sensors is much faster because the temperature element is closer to the process and the conductivity measurement relies on the temperature of the process. In addition, contacting sensors are extremely accurate at lower, near-pure water conductivities all the way to 0.01 µS/cm.
Therefore, the addition of a contacting conductivity sensor can assure fastest response to the conductivity change between clean and rinse cycles, enhancing the performance of the CIP system. Quick response time saves rinse water. The rinse water is made up of high-purity water. This water is very expensive to produce, so conserving it saves money and is better for the environment.
Contacting sensors are installed onto CIP skids and into the process piping, thus the contacting sensors are not used for measuring the process and never touch the process solution.
Offset lack of measurement experience
Conductivity measurement is so integral to the CIP process that it’s easy to ignore. Unfortunately, it’s also so critical that if it goes wrong, it can have dire effects on the process and the bottom line. Whereas the hands-on expertise of staff once might have made the measurement of conductivity a non-issue, that’s no longer true. A lot of knowledge walks out the door with every retiring engineer and technician.
For that reason, it’s important to let technology do the work by selecting analysis systems that help reduce human error. Look for analyzers that provide simple, fast setup and easy operation. Providing real-time measurement values at a glance saves time and requires no training. Some analyzers allow customization of the readout to help avoid the need to sort through unnecessary information. Most essential is an intuitive interface that provides help screens to walk personnel through processes right at the device without the need for manuals or online instructions. Most analyzers on the market can accept at least two sensors, thus lowering equipment costs as well.
Conclusion
Conductivity measurement is the backbone of CIP liquid analysis. There have been gradual changes to conductivity technology over the years, but the fundamentals remain time-honored. At the same time, making a series of smart choices and practices such as selecting for rangeability and ease of use can make a big difference in problem-free, accurate and fast clean-in-place operations that are quick and safe.
Michael Francis is global product manager at Emerson Automation Solutions.