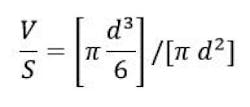Emulsion stability basics
Emulsions are found in every aspect of daily lives. The development and processing of emulsions is common in many industries. A manufacturer that uses a tooling lubricant to produce aircraft engine parts or someone applying a cosmetic cream are common examples of emulsion use.
The use of colloid mills and in-line mixers is a popular way to prepare and process emulsions. This article will discuss basic topics that will assist in the successful development and final evaluation of stable emulsions – including definition of the relevant terms, proper formulation of the product, recommended premix methods, optimization of the processing equipment and methods of evaluating the finished product quality.
Basic definitions
The logical place to begin is with a working definition of an emulsion. Many such definitions are possible, but the most basic one defines an emulsion as a stable mixture of two immiscible liquids, one of which is uniformly dispersed in the other in the form of small droplets or particles. While this seems like a simple definition, its full scope will become evident.
Since an emulsion consists of two distinct fluids, distinguishing between them is important. This is accomplished by designating the liquid that exists in the form of small droplets as the dispersed phase or internal phase. The liquid present as the surrounding medium is the external phase or continuous phase.
Based upon these distinctions, two general types of emulsions are possible. They are called oil-in-water (O/W) and water-in-oil (W/O) emulsions. In the former, the internal phase is an oil or oil miscible liquid, and the external phase is water or a water miscible liquid. In the latter, the internal phase is the water-like liquid, while the external phase is the oil-like liquid.
Most people are at least somewhat familiar with the concept of surface tension in which liquid in a container acts as if it has a skin. The molecules on the surface experience forces that tend to bind them to the bulk of the liquid and prevent their escape into the air. This is the reason that a glass of water can be carefully filled so that the surface bulges above the top of the glass without spilling.
How does this concept apply to emulsions? Consider the surface of the droplets comprising the internal phase. This surface, which represents a boundary between the two phases, is called an interface. The molecules of both phases at this interface also experience forces that tend to bind them to their own kind and make breaking a single droplet into multiple smaller droplets difficult. These forces result in an interfacial tension between the two phases. It is the activity at this interface that occupies the majority of this article.
Clearly, since the interfacial tension is a measure of the forces trying to keep the two phases separate, the goal in preparing emulsions must be to reduce the interfacial tension to promote a more intimate blending of the two phases. This is accomplished in two primary ways: By reducing the viscosity of the internal phase and through the use of chemical additives. The simplest way to achieve a viscosity reduction is to heat the product because most liquids become less viscous when they heated. The viscosity decrease is usually accompanied by a decrease in the interfacial tension, more readily making a good emulsion form.
A stable emulsion of two immiscible liquids is rare, and some type of chemical assistance is typically required. Usually, a chemical that is active at the interface between the two phases is used. Such an additive is referred to as an emulsifier or a surfactant (this stands for surface-active agent). The commercial preparation of most emulsions involves the application of both a chemical emulsifier and a mechanical device, such as a colloid mill or in-line mixer, to produce a dispersed phase with a droplet size small enough to result in a finished product with the desired properties.
A precise technical explanation of the mechanism by which emulsifiers function is beyond the scope of this discussion, but more detail will help. One can picture an emulsifier molecule as having a long tail on one end and a round head on the opposite end. The tail will be non-polar (electrically neutral) and, therefore, hydrophobic (oil soluble). The head will be polar (electrically charged) and, therefore, hydrophilic (water soluble).
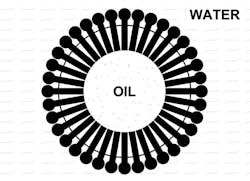
Using an O/W emulsion as an example, the emulsifier molecules will move to the interface between the phases and orient themselves in such a way that the hydrophobic ends will be embedded in the surface of the internal-phase oil droplets, while the hydrophilic ends will be bound to the continuous-phase water molecules at the interface. In effect, the emulsifier forms an interfacial film that is one molecule thick. Visually, the dispersed phase particles resemble pin cushions (see Figure 1). Of course, the orientation of the emulsifier molecules would be inverted for a W/O emulsion, but the concept is, otherwise, identical.
Figure 1. Oil-in-water emulsion interface
This molecular arrangement promotes emulsion formation and stability in two ways. First, the internal phase droplets, because they are surrounded by the electrically charged hydrophilic ends of the emulsifier molecules, are inhibited from merging to form larger droplets. In effect, they behave as electrically charged particles, and particles of like charge repel each other. Second, the breakup of these droplets will occur more easily because the interfacial tension will have been significantly lowered by the presence of the emulsifier molecules at the interface.
Physical properties
With an understanding of what emulsions are and how they are formed, some of their more important physical properties can be detailed. To simplify, only an O/W emulsion with spherical oil droplets will be covered within this article, but the conclusions equally apply to the W/O type. These properties will be featured:
- Mean particle size
- Particle size distribution
- Internal-phase viscosity
- Continuous-phase viscosity
- Emulsifier level
- Oil-phase concentration
- Continuous-phase pH
- Emulsion optical properties
The critical subject of emulsion stability is impossible to avoid. The most obvious physical characteristic of an emulsion is the size of the oil droplets. These droplets must be greater than 0.1 micrometer (µm) to avoid being classified as a colloidal suspension.
In practice, most commercial emulsions have particles ranging from about 0.2 µm to 100 µm in diameter. Because of the influence of gravity and the difference in density between the two liquids, the oil droplets will rise to the top of the container at a rate proportional to their diameter. Therefore, in the interests of emulsion quality, the oil droplet size must be reduced until the desired degree of product stability is achieved. Further, droplet size reduction beyond this point will have no practical impact and will be a waste of the energy required to achieve it.
The fact that a range of particle sizes has been mentioned implies that not all the oil droplets are the same size. A given emulsion cannot be characterized by stating a single particle size. Instead, a mean particle size, plus information on the particle size distribution, must be determined. Mean particle size can be defined in many ways, but since the total volume of the dispersed phase (V) and the total interfacial surface area (S) are such important variables in emulsion theory, a definition that incorporates these values is often used. Such a parameter is the mean volume surface diameter (dvs), and it is calculated using the standard formulas for the volume and surface area of a sphere as indicated in the equation below:
Solving for d yields the result d = 6 V/S, and this is the definition of dvs. For the remainder of this discussion, the term “mean particle size” is assumed to refer to this value.
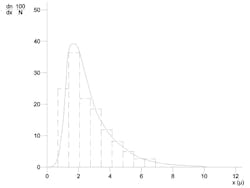
The question of distribution is usually approached by including a graphical representation of the particle size distribution curve along with the mean particle size diameter. Figure 2 shows an example of a typical log-normal particle size distribution curve, where n is the number of droplets with a diameter of x, and N is the total number of droplets of all sizes in the emulsion sample.
The concentrations in question are relevant because they influence the type and stability of the final emulsion. In general, the phase that is present in the greater concentration will tend to be the continuous phase. For example, an emulsion with 40 percent oil will tend to form an O/W emulsion. If the oil level is increased to 60 percent, it will favor the formation of a W/O emulsion. This is because it becomes increasingly difficult to pack the oil droplets into proximity without having them combine as their concentration is increased. It is easier to form water droplets and disperse them in the oil in this situation.
In fact, if oil were slowly added to the 40 percent O/W emulsion with good agitation, the emulsion would gradually become more and more viscous and then suddenly become quite fluid again. After this point is reached, closer examination would show that the emulsion was the W/O type. This process is known as inversion, and the final product is sometimes called an invert emulsion.
These parameters are useful as general guidelines, but by a careful selection of emulsifier type and strict control of the particle size to keep it from getting too small, emulsions with a very high concentration of internal phase are possible. A common example is mayonnaise, which is an O/W emulsion containing 80 percent oil. This is a situation in which the natural physical properties of the system have been overcome with much effort. We will consider such examples.
Another important variable in commercial applications is the maximum particle size. In laboratory testing of product samples, particles below a certain size separate so slowly as to be acceptable, while particles larger than that size are simply not stable enough. The average particle size would be of little concern if the tail of the distribution curve did not extend beyond that critical diameter value.
The concentration of the dispersed phase and that of the emulsifier can affect emulsion stability. First, a word of caution on terminology is needed. The concentration of the dispersed phase may be expressed as either a weight or volume percentage of the whole emulsion. The emulsifier is expressed as a weight percentage of either the total emulsion or of one phase only. All these methods are commonly used, and careful attention must be paid to these details in any mention of concentrations. In this article, both concentrations imply weight percentages of the total emulsion.
Finally, the concentration of emulsifier places a practical limit on how small an average particle size is possible. To understand why, recall that the emulsifier forms a monomolecular layer at the interface between the two phases. If the complete surface of each of the oil droplets is “covered” by emulsifier, the emulsion will be stable. However, if there is insufficient emulsifier to perform this function, the oil droplets will merge together to form larger droplets until the point of full coverage is once again reached. This phenomenon is called coalescence. When one considers that the total surface area of the oil droplets is proportional to the square of the average particle diameter, merely halving the average particle size will quadruple the concentration of emulsifier necessary to maintain the emulsion’s stability.
In some cases, a good emulsion can be produced with a moderate level of applied mechanical energy, but a poor emulsion results if the energy level is increased. The increase in applied energy causes additional particle size reduction, but without adjustment to the emulsifier concentration, the smaller particles are not stable. This is known as overworking the emulsion. Processing equipment, such as in-line mixers that offer Shear Zone Management (multiple, customizable, high-shear action zones) and Mix Order Control (adaptable mixing chambers to introduce process material at different positions in the shear zone), provides critical advantages for commercial emulsion development and processing.
The fact that a reduction in dispersed phase viscosity enhances emulsion formation has already been mentioned, but what effects can be expected from changes in the continuous-phase viscosity? Using the same argument as before, a reduction in viscosity should lead to easier emulsion formation because of a reduced interfacial tension. While this is true, another factor must be considered. An increase in continuous-phase viscosity will greatly improve emulsion stability by retarding the inevitable rise of the oil droplets to the top. In most circumstances, this more stable finished product is the overriding concern, and a decision to gain this advantage at the expense of overcoming a higher interfacial tension in the mechanical processing step is gladly accepted.
Figure 2. Log-normal distribution curve
Emulsion stability
Three distinct categories of emulsion stability must be addressed:
- Will the final emulsion type remain as O/W, or will it invert to W/O as it ages?
- The most serious instability of all is a complete separation of the emulsion into a layer of pure oil sitting on top of a layer of pure water. This demulsification, or breaking of the emulsion, would, obviously, be a catastrophe.
- By far, the most commonly encountered form of instability is creaming. Often, this is mistakenly thought to involve the formation of an oil layer on top of the emulsion. However, it is the separation of the initial emulsion into two emulsions. The one at the top of the container has a much higher internal phase concentration than the original emulsion. The one at the bottom of the container has a much lower internal phase concentration than it originally had.
Stability regarding emulsion type has been partially addressed. Recall that the dispersed phase concentration plays an important role. To minimize the chances for a gradual inversion, the oil concentration should be kept as far below the level at which complete inversion occurs as practical. The specific emulsifier choice is critical. Generally, the phase in which the emulsifier has the greater solubility tends to be the external phase. If one wishes to make an O/W emulsion, the emulsifier selected should have a somewhat greater solubility in water than in oil.
These variations in emulsifier solubility are governed by the relative sizes of the hydrophilic and hydrophobic ends of the molecule. An interesting phenomenon relating to this topic can occur if the wrong formulation choices are made. A double emulsion can result. In this case, it would be a water-in-oil-in-water emulsion. Microscopic observation would show the expected oil droplets emulsified in the water, but water droplets would also be emulsified into each of those oil droplets. A situation like this is likely to result if the two phases are present in roughly equal volumes and the emulsifier has almost equal solubility in oil and water.
A complete breaking of the emulsion normally occurs in two stages. First, the dispersed phase droplets form aggregates, and the droplets become attached to each other without losing their individual identity. This process, called flocculation, destabilizes the emulsion because the large aggregates separate out more rapidly than the smaller individual droplets. However, the process is usually reversible upon application of agitation and/or the addition of a suitable surfactant. On the other hand, coalescence, the second stage of demulsification is much more serious. This is a non-reversible process in which several internal phase droplets merge together into a single large droplet.
Creaming is the most commonly encountered type of emulsion instability. It results when the inexorable work of the gravitational forces causes two liquids of different densities to stratify. This effect can be slowed by the techniques discussed in this article, but it cannot be stopped. Therefore, the real concern should be the rate of creaming. It is acknowledged that creaming will occur, but it must be slowed to a tolerable rate.
Types
Before discussing emulsion processing and evaluation, the emulsifier types must be defined. The surface-active emulsifiers, with which most processors are primarily concerned, can be subdivided into anionic, cationic, nonionic and amphoteric. These terms refer to the electrical charge that the emulsifier imparts to the dispersed-phase particles:
- Anionic = negative charge
- Cationic = positive charge
- Nonionic = electrically neutral
- Amphoteric = pH-dependent charge
The influence on emulsion stability of some important external factors depends on the electrical characteristics created by the emulsifier. For example, anionic types are effective at high pH levels, while cationic types are effective at low pH levels. The nonionic types are relatively insensitive to the emulsion’s pH.
Similarly, the stability of emulsions formed with electrically charged surfactant molecules is much more likely to be disturbed by the addition of electrolytes to the finished product. This is the reason that the addition of a trace amount of a salt often destroys some emulsions. When contact with electrolytes is expected, a nonionic emulsifier type should be used.
Processing methods
To create an emulsion, the ingredients are first combined to form a crude premix emulsion. This premix can be created in several ways:
- The emulsifier is dissolved in the continuous phase, and then the internal phase is slowly added with good agitation (most common method).
- The emulsifier can be dissolved in the internal phase before slowly adding that blend to the continuous phase under agitation.
- The emulsifier can be dissolved in the internal phase before slowly adding the continuous phase to form the premix. This means usually produces the best results, but it requires a lot of time and vigorous mixing because it involves bringing a preliminary W/O emulsion through the inversion stage to eventually form the desired O/W type.
- Another method is using a mix-order control method specifically developed by Bematek. This technique permits the injection of the product components directly into the product stream at different steps along a multistage mixing chamber.
If a mechanical shearing device such as a colloid mill or an in-line mixer is used in the finishing step, the first premix method usually produces good results.
One frequently used premix option must be mentioned separately. Many O/W emulsions are stabilized with simple soaps. In these situations, the best results are obtained by dissolving the fatty acid part of the soap in the oil phase and the alkaline part of the soap in the water phase. Then the oil is added to the water with agitation. The resulting formation of the soap directly at the interface as the two phases are combined leads to an excellent premix. Note that an in-line mixer with mix-order control ability can accomplish the simple-soap method in a continuous, single-pass system instead of using a premix tank.
Having assured a well-formulated and stable premix, the colloid mill or in-line mixer can finish the job. The zone of intense hydraulic shear forces within the colloid mill or in-line mixer head breaks the internal phase droplets apart and creates the small particle size that is generally desired. If a sufficient emulsifier is used for the enormous increase in surface area generated by this process, the final product should exhibit enhanced stability.
After preparing the emulsion, it is key to find a way to measure its quality so that some degree of consistency from one batch to another can be maintained.
Quality control
With the emulsions’ physical properties, the information to verify the results with a reliable quality control (QC) process is available. The easiest method is to put the emulsion on a shelf and observe it for creaming over time. A minimum acceptable shelf life can be a QC specification. Unfortunately, the price for this simplicity is that a poor batch might not be discovered until after the product reaches the customer. To overcome this, the creaming process can be accelerated by heating the emulsion or by centrifuging it. These results must then be related to a corresponding static creaming rate at room temperature. All these creaming-rate measurements are simple, but they are not precise.
The creaming rate of an emulsion is directly related to the size of the dispersed phase droplets. Therefore, measuring the droplet size indirectly provides the desired stability information. These methods include:
- An optical microscope for observing the maximum particle size present
- Measurements of the percentage of light transmitted (the emulsion becomes more transparent as the particle size is reduced)
- Light-scattering measurements for generating detailed particle size distribution curves
These methods are quick and accurate. After a relationship between particle size and shelf life has been established, precise predictions of the creaming rate can be made from the particle size analysis.
Another useful technique is that of viscosity measurement. Many emulsions will undergo a substantial viscosity increase as the droplet size is reduced. The amount of this increase will, therefore, be a good indicator of emulsion quality. For this reason, a viscosity measurement obtained with a device such as a Brookfield viscometer can provide an excellent QC benchmark in such cases. Many other techniques are used in specific cases. For example, cosmetic emulsions are often required to withstand several freeze/thaw cycles and to be stable at elevated temperatures before they are considered acceptable.
Conclusion
This article only covers the most basic concepts of this complex and wide-ranging topic. Many books are available that go into detail on these topics and many others.
Stephen F. Masucci is technical director for Bematek. He has been involved in the formulation, scale-up, processing and testing of emulsions and dispersions since 1973.
Chris Little is a senior manager for Bematek, and with 25 years of facility and process industry experience, he is considered an expert in process improvement and equipment reliability.
Bematek